Description
SAFETY DATA SHEET
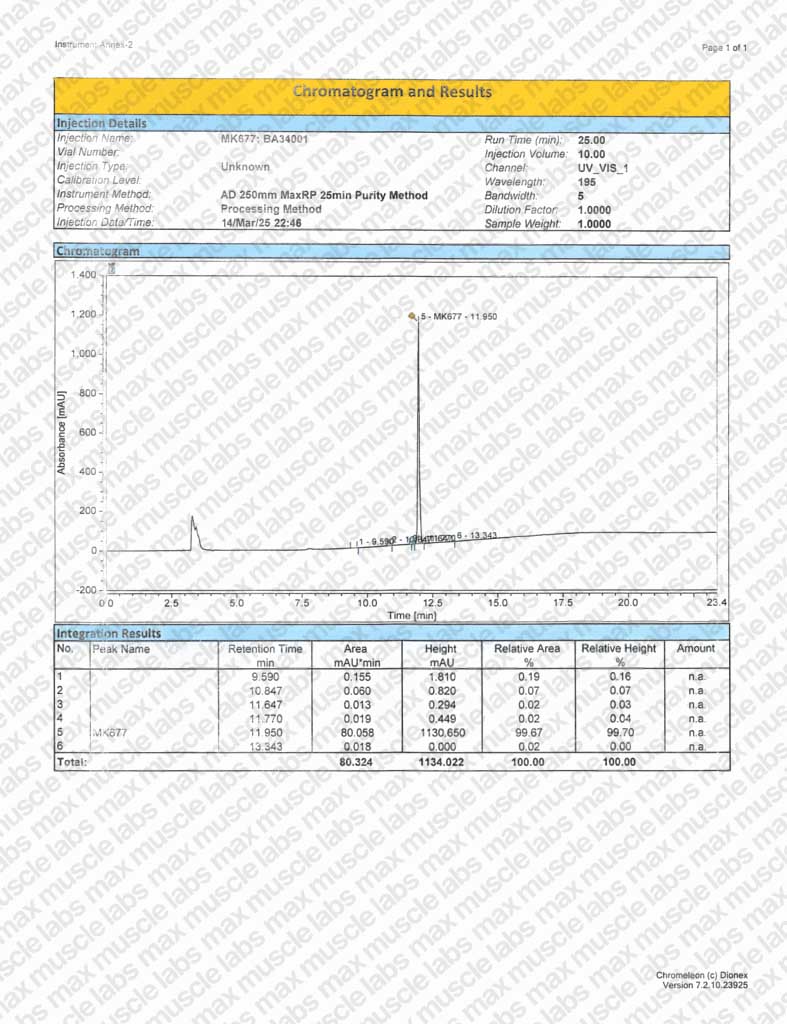
MK-677 HPLC BA34001
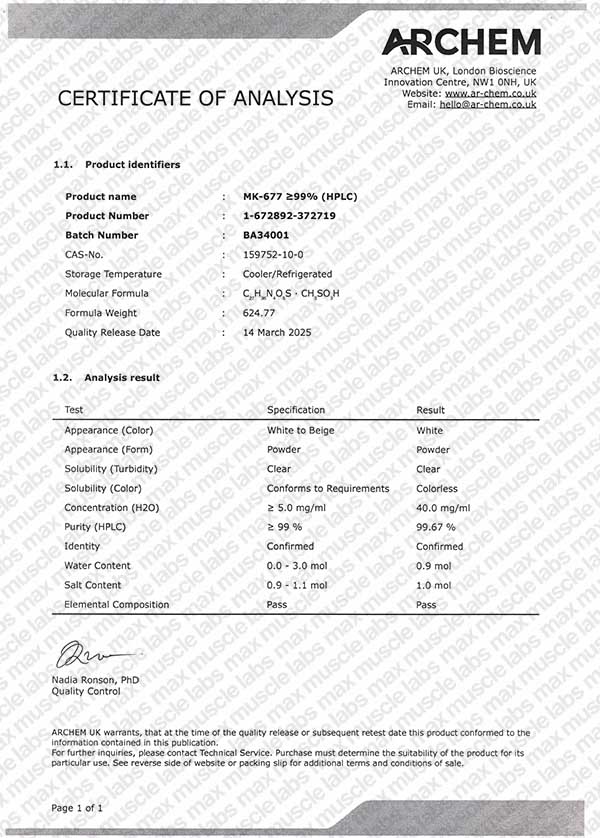
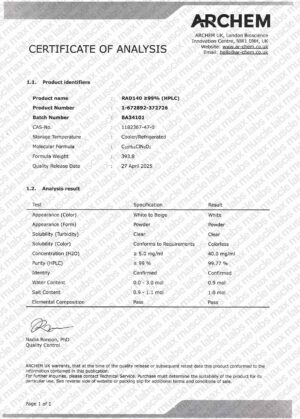
MK-677 COA BA34001
Core learning : What you need to know about MK677
Ibutamoren, also known as MK-677, is an orally active, long-acting growth hormone secretagogue that mimics the action of ghrelin by binding to the ghrelin receptor (GHS-R1a) in the brain. This results in a sustained, dose-dependent increase in circulating growth hormone (GH) and insulin-like growth factor 1 (IGF-1) levels without significantly affecting cortisol or prolactin secretion. Unlike recombinant GH administration, MK-677 stimulates natural pulsatile release of growth hormone, which may preserve physiological feedback mechanisms and receptor sensitivity.
In clinical and experimental settings, MK-677 has demonstrated the ability to:
- Facilitate increases in fat-free mass and enhances nitrogen balance in preclinical models
- Modulate IGF-1-mediated signaling pathways implicated in skeletal muscle regeneration and tissue repair
- Demonstrate potential to influence bone mineral density parameters, particularly in age-related research contexts
- Affect sleep architecture and REM phase duration, likely through central ghrelin receptor (GHS-R1a) activation
- Alter gene expression and signaling cascades associated with dermal, follicular, and extracellular matrix dynamics in regenerative biology models
Ibutamoren’s oral bioavailability, extended half-life, and minimal suppression of endogenous hormone production make it an attractive candidate for long-term research into GH-related therapies, sarcopenia, and age-associated decline in anabolic hormones.
CAS Number: 159752-10-0
Molecular formula: C27H36N4O5S
Molar mass: 528.7 g/mol
Purity: ≥99% (see HPLC and COA documentation)
Concentration: 10 mg
Half-life: ~24 hours
Storage: 2 years at 20°C
⚠ Important Note: This product is supplied strictly for laboratory research use only. It is not approved for human consumption or therapeutic use. Not intended to diagnose, treat, cure, or prevent any disease.
What Is MK-677 (Ibutamoren)?
A non-peptide growth hormone secretagogue used in investigational studies on GH axis modulation, aging, and muscle wasting.
MK-677, not to be confused with MK-2866 (Ostarine) also known by its research code Ibutamoren mesylate, is a selective, orally active growth hormone secretagogue (GHS). It is not a SARM (Selective Androgen Receptor Modulator) but is frequently grouped with them due to its presence in performance-enhancement and metabolic studies.
🔬 Mechanism of Action

MK-677 functions as a ghrelin receptor agonist — specifically targeting the GHS-R1a (Growth Hormone Secretagogue Receptor 1a) located in the hypothalamus and pituitary.
Once bound:
- It mimics endogenous ghrelin, stimulating pulsatile GH release
- This cascade leads to elevated IGF-1 levels, a key mediator of tissue growth and recovery
- Unlike traditional GH therapies, MK-677 does not suppress endogenous hormone production
“In contrast to exogenous GH, MK-677 stimulates physiological GH release, preserving hypothalamic–pituitary signaling.”
— Murphy et al., J Clin Endocrinol Metab, 1998
📊 Key Research Findings
| Study Domain | Findings |
|---|---|
| GH/IGF-1 axis | Increases GH and IGF-1 levels dose-dependently (e.g., 1–3 mg/kg) |
| Body composition | Promotes lean body mass retention and slight fat loss in clinical studies |
| Muscle preservation | Shown to mitigate age-related sarcopenia and improve muscle strength |
| Bone density | May enhance bone turnover markers via IGF-1 activity |
| Sleep studies | Improves REM sleep duration and sleep quality metrics |
⚠️ Important Notes for Research Use
- Not approved for human use outside of clinical trials
- Typically studied in double-blind, placebo-controlled settings
- Pharmacokinetics show a long half-life (~24 hours) and oral bioavailability
- Some studies report transient increases in blood glucose and appetite
“MK-677 is a promising compound for long-term GH/IGF-1 modulation, but requires careful metabolic monitoring in trial models.”
— Smith et al., Growth Horm IGF Res, 2000
🧪 Typical Research Use Parameters
| Form | Oral suspension / powder (research-grade) |
|---|---|
| Dose Range (animal/lab) | 5–25 mg/day depending on model size and metabolism |
| Study Duration | 2–12 weeks in preclinical and human studies |
| Detection Method | LC-MS/MS for plasma analysis; GH and IGF-1 serum assays |
Summary & Further reading
Read our articles:





Rob (verified purchase) –
Great product. I recommend it!
Tristan (verified purchase) –
packaging and shipping was great. excellent research results.
Sam (verified purchase) –
strong
Liam (verified purchase) –
Seems reliable
John (verified purchase) –
so far so good will come back for more
Tom (verified purchase) –
This product is legit. Great experience.
Darren (verified purchase) –
good quality sarms
William (verified purchase) –
great ,so good, thans so much for everything
Harry (verified purchase) –
Works
Nathan (verified purchase) –
Quality products delivered quickly.
Ivan (verified purchase) –
Awesome. It arrived in Czechia in 5 days. I had no problems whatsoever 10/10
Gary (verified purchase) –
Works well
Chris (verified purchase) –
After being unsure and doing a lot of reading, I found this website, and I’m very happy that I did. I’m glad to know there is somewhere I can go to feel confident about this kind of product. Tnx for communicating with me through the process.
Richard (verified purchase) –
Best value (price and quality) on the internet
david (verified purchase) –
I’m very impressed with this material. Thank you!
collin (verified purchase) –
legit 100%
Jim (verified purchase) –
Valid
Shawn (verified purchase) –
Kinda surprised me to be honest quality is on point
Philip (verified purchase) –
Good
David (verified purchase) –
Accurate description. Conforms to chemical specifications.
Bill (verified purchase) –
Awesome, worked just as I expected
Scott (verified purchase) –
It’s definitely one of the best out there. This has to be the purest as I haven’t come across these effects in research from other companies yet.
Albert (verified purchase) –
ok
MML Support (store manager) –
Hi Albert,
Thanks for your feedback — we appreciate you taking the time to leave a review.
If there’s anything we can do to make your experience a 5-star one next time, feel free to let us know!
Thomas (verified purchase) –
I actually like the result
Alex (verified purchase) –
quick delivery
Nick (verified purchase) –
I purchased MK677 about two weeks ago. It arrived quickly. The quality is excellent.
andrew (verified purchase) –
Getting great results
Dennis (verified purchase) –
Fast delivery Priced reasonable
james (verified purchase) –
great
Cory (verified purchase) –
Extremely fast service thank you
Eric (verified purchase) –
Good stuff. Definitely will order from them again.
Kenneth (verified purchase) –
I checked the lab reports and they seem legit
Robert (verified purchase) –
Really high quality
Jordan (verified purchase) –
Im impressed with how good and effective MMS/Archem RAD-140 and MK-677 are. They work well and you can rely on them, so its a good choice for research