The gold standard for verifying compound purity, safety, and identity.
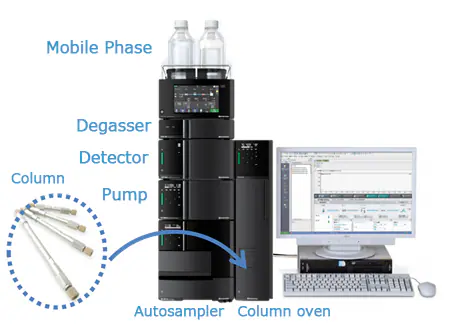
When it comes to SARMs, labelling isn’t enough — you need data. That’s where HPLC comes in.
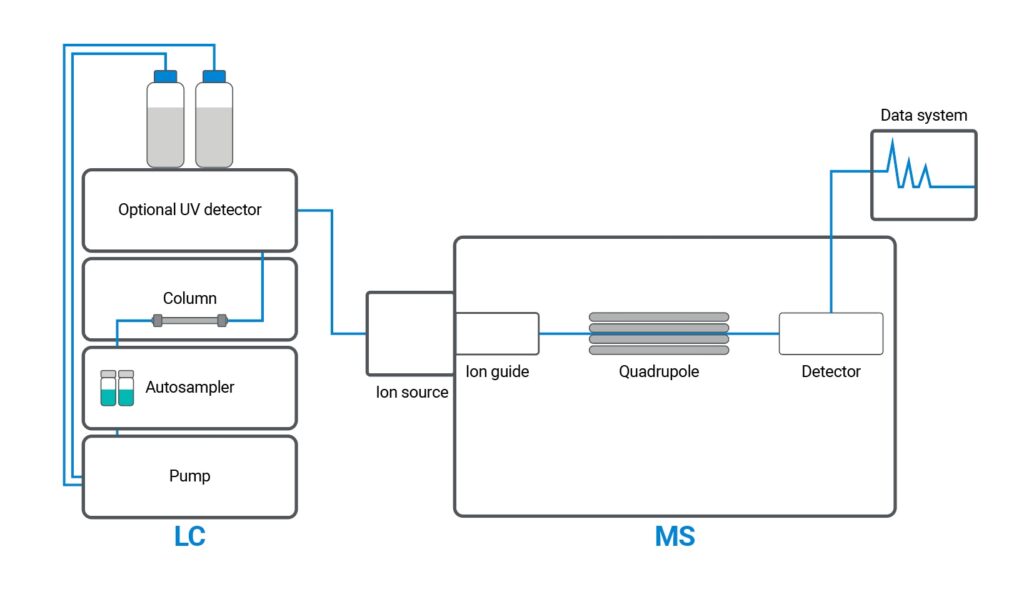
High-Performance Liquid Chromatography (HPLC) is the industry-standard analytical method used to verify the purity, identity, and stability of chemical compounds, including Selective Androgen Receptor Modulators (SARMs). It’s trusted by pharmaceutical labs, regulatory bodies, and legitimate suppliers for a reason: it’s fast, precise, and reproducible.
Let’s break down how it works — and why it matters.
🧪 What Does HPLC Actually Do?
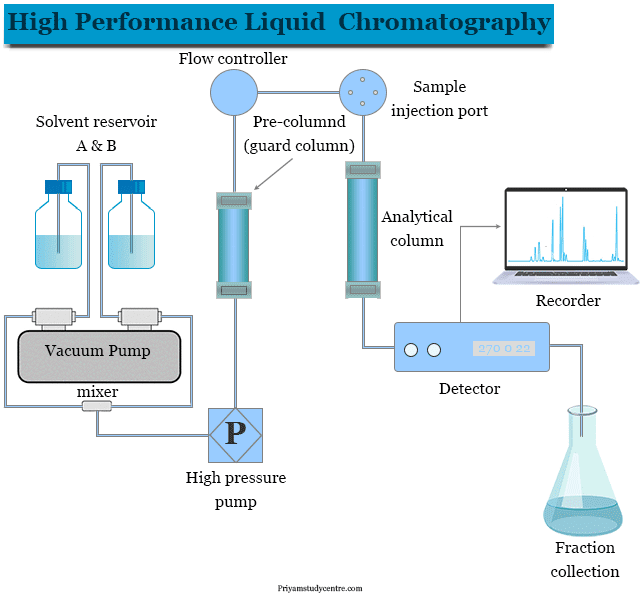
HPLC separates the chemical components in a sample and detects exactly what’s present — and in what concentration.
Here’s the process in brief:
- A liquid solvent (the “mobile phase”) pushes the SARMs sample through a column packed with a solid material (the “stationary phase”).
- As compounds pass through the column, they separate based on molecular characteristics like size, polarity, and interaction time.
- A detector then captures the results, outputting a chromatogram: a visual chart showing distinct peaks for each substance in the sample.
Each peak represents a different compound — and its position, height, and shape reveal its identity and concentration.
✅ Why HPLC Is Essential for SARMs Testing
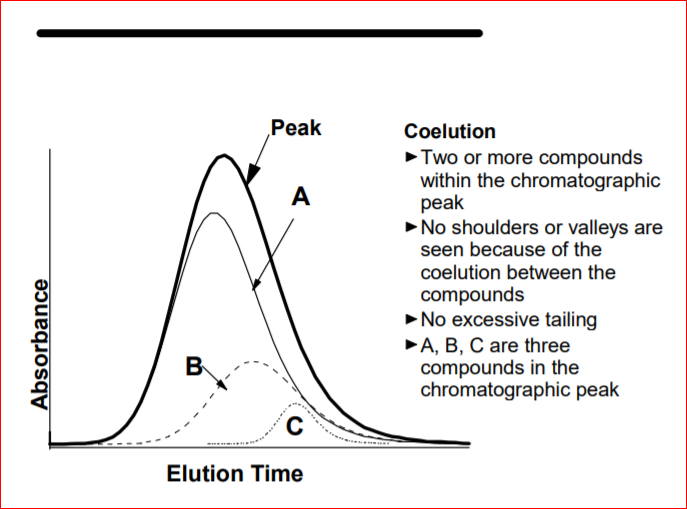
1. Purity Confirmation
HPLC tells you whether a compound is 98% pure or 70% filler. This is critical in a space where under-dosed, mislabeled, or contaminated products are common.
“Without HPLC verification, you’re trusting the label — not the lab.”
— Independent Quality Control Analyst, SARMs UK Forum
✅ What Does “Purity” Mean in SARMs Testing?
Purity is the percentage of the sample that consists of the target compound, without contaminants, synthesis byproducts, solvents, or incorrect isomers.
- A 98% pure RAD-140 sample means 98% of the content is RAD-140, and 2% is other substances.
- A low-purity product might contain fillers, degradation products, or worse — entirely different compounds (like prohormones or steroids).
This isn’t just about quality — it’s about whether your data is valid or contaminated by noise.
2. Batch-to-Batch Consistency
HPLC allows suppliers to compare one batch to the next — ensuring consistency across production runs, which is essential for reproducible research outcomes.
3. Contaminant Detection
By identifying unexpected peaks in the chromatogram, HPLC can uncover:
- Unlisted prohormones
- Synthesis byproducts
- Toxic residues or solvents
This protects researchers from false data — and from safety risks.
🔬 How Contaminants Are Detected in SARMs
Legitimate suppliers use a combination of analytical techniques to ensure no contaminants are present in the final product. The most trusted methods include:
1. HPLC (High-Performance Liquid Chromatography)
HPLC doesn’t just confirm purity — it also flags anything that shouldn’t be there.
- Unexpected peaks in the chromatogram indicate unknown or unwanted compounds
- Even minor contaminants (≤1%) show up with precise retention times
- Ideal for spotting unreacted chemicals, isomers, or additives
2. LC-MS/MS (Liquid Chromatography + Tandem Mass Spectrometry)
This combines separation (LC) with mass detection (MS) to confirm what each compound is — not just that it’s “different.”
- Excellent for identifying molecular weight and structure of unknowns
- Often used to detect banned steroids or prohormones in contaminated SARMs
- Capable of sub-ppm (parts per million) sensitivity — essential for clean data
3. ICP-MS (Inductively Coupled Plasma Mass Spectrometry)
Used for heavy metal screening — think lead, mercury, cadmium, arsenic.
- Detects toxic trace elements in the microgram or nanogram range
- Ensures compliance with safety standards (especially important in pharmaceutical settings)
4. GC-MS (Gas Chromatography + Mass Spectrometry)
Mainly used to detect residual solvents or volatile organic compounds (VOCs).
- Identifies leftover cleaning agents or incomplete solvent evaporation
- Essential for confirming compound safety and stability
🚩 How Contamination Harms Research
Even small amounts of contamination can cause:
- False-positive results in receptor binding or anabolic activity
- Inaccurate pharmacokinetics due to reactive byproducts
- Cytotoxicity in in vitro studies
- Violations of research ethics or compliance protocols
“We once detected a testosterone ester in a sample labelled as RAD-140 — the supplier didn’t respond, and the entire study had to be scrapped.”
— Juliette Hawkins – University of Cambridge
When you’re working with SARMs such as MK-677 or Ostarine in a lab setting, purity isn’t the only concern. Contaminants — even in trace amounts — can interfere with results, introduce unknown variables, and put your research (or reputation) at risk.
That’s why contaminant detection is a critical step in any SARMs quality control process — especially for compounds being sold for analytical or experimental use.
Let’s explore what types of contamination can occur, how they’re detected, and what makes a reliable supplier stand out.
⚠️ Types of Contaminants in SARMs
Contaminants can enter a compound at multiple stages: synthesis, storage, or packaging. Here’s what to watch for:
| Contaminant Type | Why It Matters |
|---|---|
| Unreacted precursors | Leftover materials from the synthesis process can interfere with results |
| Byproducts & isomers | Structural variations that behave differently in biological assays |
| Prohormones or steroids | Sometimes added intentionally by shady suppliers to simulate effects — illegal risk |
| Solvent residues | Toxic or reactive solvents (e.g., DMSO, acetone) from incomplete drying |
| Heavy metals | Introduced via raw materials or contaminated equipment — highly toxic in micrograms |
| Moisture or degradation | Leads to instability, reduced efficacy, or unpredictable results |
4. Verification of Identity
Each compound has a unique retention time — its “fingerprint” on the chromatogram. HPLC confirms that what’s sold as RAD-140 is, in fact, RAD-140 — not a cheaper or misidentified compound.
🔍 Why HPLC > In-House or Visual Testing
Some vendors claim “lab tested” but only perform basic in-house checks or color reactions. These cannot detect impurities at a molecular level — and certainly can’t provide quantifiable purity metrics.
HPLC, on the other hand, offers:
- Precise, quantitative data (typically accurate to 0.01%)
- Regulatory-grade results used by pharmaceutical companies
- Traceable records that can be linked to batch numbers and COAs
If your SARMs supplier isn’t using HPLC or LC-MS/MS — or refuses to show the results — that’s a serious red flag.
📊 HPLC Retention Times of Common SARMs (Analytical Reference Table)
| SARM Compound | Approx. Retention Time (min) | Detection Wavelength (nm) | Notes |
|---|---|---|---|
| Ostarine (MK-2866) | ~7.0 – 8.5 min | 265–280 nm | Sharp peak, low polarity |
| Ligandrol (LGD-4033) | ~5.5 – 6.5 min | 254 nm | Retains well on C18 columns |
| RAD-140 (Testolone) | ~9.5 – 11.0 min | 254 nm | Often delayed due to strong aromatic groups |
| YK-11 | ~8.0 – 9.5 min | 265–280 nm | Similar profile to steroids, requires clean baseline |
| S-23 | ~6.0 – 7.5 min | 260 nm | Polar structure, moderate elution |
| ACP-105 | ~4.5 – 6.0 min | 254 nm | Lower retention due to polarity |
| MK-677 (Ibutamoren) | ~10.5 – 12.0 min | 254 nm | Peaks slightly broader, multiple conformers possible |
| SR9009 (Stenabolic) | ~5.0 – 6.0 min | 270 nm | May co-elute with byproducts if not pure |
| SR9011 | ~5.5 – 6.5 min | 270 nm | Similar to SR9009, lower intensity signal |
| GW501516 (Cardarine) | ~11.0 – 12.5 min | 265–280 nm | Long retention, aromatic and lipophilic |
| Andarine (S4) | ~6.5 – 7.5 min | 254 nm | Often exhibits minor impurity peaks |
| RAD-150 | ~9.0 – 10.5 min | 254 nm | Longer elution vs RAD-140 due to ester group |
⚠️ Important Notes:
- Retention times assume reverse-phase HPLC using a C18 column, with a typical acetonitrile/water gradient.
- Detection is most commonly performed using UV-Vis DAD at 254–280 nm, depending on the compound’s chromophores.
Final Word
In a market where quality control is often claimed but rarely proven, HPLC separates legitimate suppliers from the rest.
If you’re conducting serious research, demand:
- Batch-specific HPLC results
- A visible, readable COA (Certificate of Analysis)
- Full documentation showing testing method, lab name, and date
- Read our SARMs buying guide
📚 References for HPLC Analysis of SARMs
1. Deventer, K., et al. (2015).
“Screening for SARMs in urine: Analytical challenges and detection strategies.”
Drug Testing and Analysis, 7(11–12), 1061–1071.
https://doi.org/10.1002/dta.1842
This paper outlines HPLC and LC-MS/MS detection protocols for several SARMs, including RAD-140, Ostarine, and LGD-4033.
2. Thevis, M., et al. (2013).
“Mass spectrometric determination of selective androgen receptor modulators.”
Analytical and Bioanalytical Chemistry, 405(27), 9017–9027.
https://doi.org/10.1007/s00216-013-7256-z
Includes detailed retention time and fragmentation patterns for SARMs using LC-HRMS.
3. World Anti-Doping Agency (WADA)
“SARMs and non-approved substances: Analytical methods in doping control.”
WADA Technical Documents and Prohibited List (2023 edition):
https://www.wada-ama.org
Provides standardized protocols for detecting SARMs in biological matrices using HPLC and tandem MS.
4. European Pharmacopoeia Monographs (EDQM)
When available, pharmaceutical-grade SARMs tested for research often follow methods outlined in EP monographs. These include:
- UV-Vis detection wavelengths
- Retention time expectations
- Purity thresholds
Check: https://www.edqm.eu
