Ostarine (MK-2866): What Researchers Need to Know
1. Overview
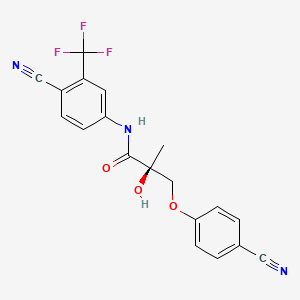
Ostarine — often referred to as MK-2866 or Enobosarm — is a Selective Androgen Receptor Modulator (SARM) developed by GTx, Inc. for conditions like muscle wasting and osteoporosis.
“Ostarine represents one of the most clinically advanced SARMs, with multiple Phase II trials conducted in cancer-related cachexia and age-related sarcopenia.” – Journal of Cachexia, Sarcopenia and Muscle
Unlike anabolic steroids, Ostarine was designed to provide anabolic effects in muscle and bone without strongly affecting tissues such as the prostate.
📊 Table 1: Core Chemical & Research Attributes of Ostarine (MK-2866)
| Attribute | Detail |
|---|---|
| Research Code | MK-2866 |
| Trade Name | Enobosarm |
| Compound Class | Non-steroidal aryl propionamide SARM |
| Molecular Formula | C₁₉H₁₄F₃N₃O₃ |
| Molecular Weight | ~389.33 g/mol |
| Form | Solid, white to off-white powder |
| Solubility | Soluble in DMSO, ethanol, PEG solvents |
| Stability | Stable under −20°C; protect from light and moisture |
| Primary Research Use | Muscle wasting, sarcopenia, osteoporosis studies |
| Clinical Status | Phase II human trials completed; no current regulatory approval |
| WADA Status | Prohibited substance under Anabolic Agents category |
| Storage Guidance | Store at −20°C, sealed container, desiccated environment |
2. Mechanism of Action
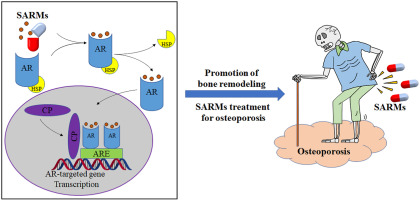
The research appeal of Ostarine lies in its selectivity:
- ✅ Receptor-specific binding: Stimulates protein synthesis and muscle hypertrophy.
- ✅ Tissue-targeted effects: Promotes lean mass without pronounced androgenic side effects.
- ✅ Model compound: Frequently cited in literature for studying androgen pathways and the biology of muscle preservation.
“SARMs such as Enobosarm are capable of mimicking testosterone’s anabolic benefits while limiting unwanted androgenic activity.” – Endocrine Reviews
3. Research Applications
Clinical research has explored Ostarine for:
- Cancer cachexia: Phase II trials showed measurable improvements in lean body mass and physical performance.
- Sarcopenia (age-related muscle decline): Demonstrated anabolic benefits in older populations without typical steroid complications.
- Osteoporosis & rehabilitation: Investigated for its bone-preserving potential.
📌 Important: Ostarine is not approved for medical use. All applications remain strictly investigational.
Table 2: Key Clinical Studies of Ostarine (Enobosarm / MK-2866)
| Study Focus | Title & Details | Link |
|---|---|---|
| 1. Elderly / Postmenopausal (Phase II) | The selective androgen receptor modulator GTx‑024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women – Phase II, double‑blind, placebo‑controlled trial showing dose‑dependent increases in lean body mass and physical function PubMed | [PubMed – Dalton et al. 2011] |
| 2. Cancer Cachexia (Phase II) | Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial – Improvements in lean mass and stair-climb power in cancer-induced muscle wasting PubMed | [PubMed – Dobs et al. 2013] |
| 3. Advanced Breast Cancer (Phase II) | Activity and safety of enobosarm … in androgen receptor-positive, estrogen receptor-positive, HER2-negative advanced breast cancer – Phase II, showing anti-tumor activity and safety profile PubMedThe Lancet | [Lancet Oncology – Palmieri et al. 2024] |
| 4. Weight-Loss + GLP-1 Agonist (Phase IIb) | Veru’s Phase 2b QUALITY study: Enobosarm preserved lean mass and improved body composition in patients receiving WEGOVY® (semaglutide) for weight reduction Veru Inc. | [Veru Pharma Press Release – 2025] |
4. Safety & Legal Context

- 🚫 Approval status: Not licensed by the FDA, EMA, or MHRA.
- 📦 Research-only supply: Legally sold in the UK as a research chemical (must comply with REACH and laboratory handling standards).
- 🏅 Sporting ban: Listed on the b.
Ostarine was originally developed to help patients with muscle wasting, cachexia, and osteoporosis. In controlled trials, it showed measurable benefits in lean mass and function.
“Ostarine increased lean body mass and improved stair climb power in a dose-dependent manner, with good overall tolerability.” – Dalton et al., 2011, Journal of Cachexia, Sarcopenia and Muscle [DOI: 10.1007/s13539-011-0019-z]
However, these studies were short-term (typically 12 weeks or less) and conducted under strict medical supervision. Long-term safety data in humans is not available.
While Ostarine is often marketed as a “safer alternative” to anabolic steroids, it is not risk-free. Documented or suspected side effects include:
- Hormonal suppression: decreased natural testosterone production
- Lipid profile changes: reduced HDL (“good” cholesterol)
- Liver enzyme elevations in some reports
- Unknown long-term effects due to lack of large-scale studies
“Although SARMs such as ostarine are promoted as safer than steroids, they are not without risks, including hepatotoxicity and endocrine disruption.” – U.S. Food & Drug Administration (FDA), 2017 warning letter
Table 3: Legal Status of Ostarine (Enobosarm / MK-2866) by Country
| Country | Legal Status Summary |
|---|---|
| United States | Considered an unapproved investigational drug; cannot be marketed as a dietary supplement or drug. Also prohibited in sports. |
| United Kingdom | Not approved for human consumption; though widely accessible online, it remains illicit. |
| General (Global View) | Classified as an investigational drug and not approved globally. It’s also listed as a banned substance in competitive sports under WADA’s Anabolic Agents category. |
| US Military / DoD (Implication) | SARMs like Ostarine are considered unapproved drugs, illegal as dietary supplements, and prohibited for military service members. |
| United States (Medical Context) | Remains an investigational agent, with no FDA approval for any medical use, but still appears in some dietary supplements despite regulations. |
“Ostarine (Enobosarm) is prohibited at all times (in- and out-of-competition) as an anabolic agent.” – WADA, 2025
5. Key Takeaways for Researchers

- Ostarine = nonsteroidal SARM with muscle- and bone-specific effects.
- Considered a reference compound for studying anabolic pathways.
- Favorable short-term trial data, but long-term human safety remains uncertain.
- Legal acquisition requires research compliance with chemical regulations.
📊 Ostarine (MK-2866) at a Glance
| Property | Detail |
|---|---|
| Code Name | MK-2866 / Enobosarm |
| Compound Type | Nonsteroidal SARM |
| Primary Research Use | Muscle wasting, osteoporosis, sarcopenia |
| Mechanism | Selective AR binding → lean mass & bone preservation |
| Clinical Development | Phase II trials (cachexia, sarcopenia) |
| Legal Status (UK) | Not approved for human use, sold as research chemical |
| WADA Status | Prohibited substance |
