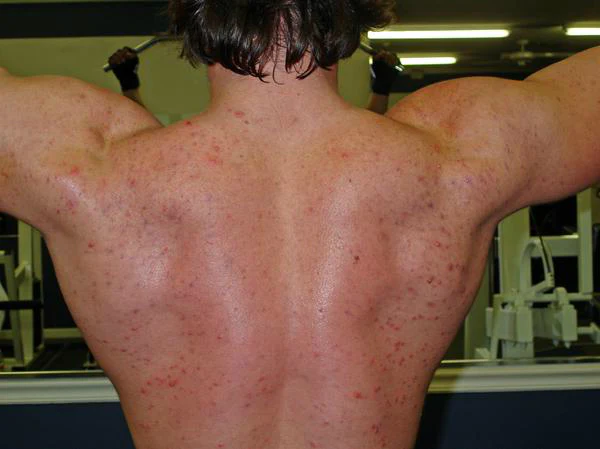
Acne is one of the most common side effects associated with androgen-modulating compounds — but what about SARMs?
With selective androgen receptor modulators gaining traction in clinical and preclinical research, a frequent question arises: Do SARMs cause acne in the same way anabolic steroids do?
The short answer: some SARMs have been associated with acneiform eruptions in clinical studies, but the mechanism appears to differ slightly from that of traditional androgens like testosterone or DHT.
This article explores the evidence from authorised trials, proposed mechanisms, and what researchers need to consider when studying SARMs’ dermatological effects.
🔬 Key Takeaways
- Acne has been reported in multiple SARMs clinical trials, particularly with compounds like Ostarine and Ligandrol
- The androgen receptor (AR) plays a central role in sebum production, inflammation, and follicular activity
- Unlike testosterone, SARMs do not convert to DHT or estrogen, which may alter their acne profile
- Dosage, compound selectivity, and study duration appear to influence acne incidence
- Research remains limited — further dermatological evaluation is needed
SARMs and Acne: What’s the Connection?
SARMs work by binding selectively to androgen receptors in muscle and bone tissue — but these receptors also exist in sebaceous glands, hair follicles, and skin keratinocytes.
🧠 “Androgens influence sebaceous gland size and activity, which directly contributes to acne pathogenesis,” notes Dr. Julie Harper, President of the American Acne & Rosacea Society. “Even mild modulation of these receptors can lead to increased oil production and inflammation.”
This suggests a biological plausibility for SARMs influencing acne — even if they don’t elevate DHT, which is strongly linked to acne in traditional anabolic steroid users.
What Do Clinical Trials Show?

Ostarine (MK-2866)
In a Phase II study on elderly men with muscle wasting, acneiform eruptions were reported in a small percentage of subjects, especially at higher doses (3 mg+) .
“The incidence of androgen-related side effects was low but included acne and headache in the treatment arms,” the trial investigators noted.
While the rate was lower than with anabolic steroids or testosterone therapy, it confirmed dermatological changes as non-zero and dose-dependent.
Ligandrol (LGD-4033)
Ligandrol was studied in a 21-day trial with healthy men at doses from 0.1 to 1.0 mg. Acne was observed in multiple participants, particularly in the higher-dose groups .
“Acne and dry skin were the most frequently reported skin-related events,” according to the study authors. “These events were self-limited and did not result in discontinuation.”
Again, while mild and transient, acne was clearly documented, aligning with androgenic modulation of sebaceous activity.
RAD-140 and Others
Data on RAD-140, YK-11, and S-23 is mostly preclinical, with limited human dermatological reporting. However, anecdotal feedback from ongoing research often includes mentions of acne as a common occurrence, especially in studies involving supra-physiological dosages.
In preclinical models, RAD-140 has shown strong AR agonism — suggesting that androgen-sensitive tissues, including skin, could be affected [4].
The Mechanism: How SARMs Might Trigger Acne
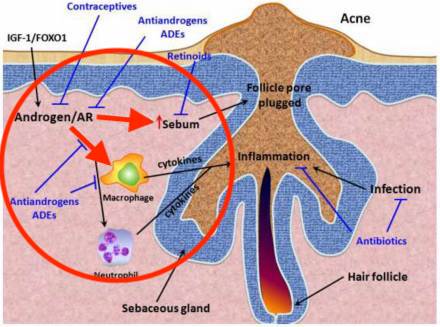
While SARMs do not aromatize or convert to DHT, their direct activation of ARs in sebaceous glands appears sufficient to trigger acne in susceptible individuals.
Here’s how the cascade works:
- SARMs bind to ARs in sebaceous glands
- This may increase sebum production (oil on skin)
- Excess sebum creates a hospitable environment for Cutibacterium acnes (C. acnes)
- Pores clog, inflammation develops, leading to papules, pustules, or cysts
🧪 “Even without conversion to DHT, direct AR activation can upregulate lipogenesis in sebaceous glands,” explains Dr. Tamara Hillman, a clinical dermatologist. “This is particularly true when tissue selectivity is less than perfect.”
This aligns with the mild but present acne rates seen in several trials.
Why SARMs May Cause Less Acne Than Steroids
SARMs are designed to be tissue-selective — ideally targeting muscle and bone while sparing the skin, prostate, and other androgen-sensitive tissues.
Unlike exogenous testosterone or DHT-derivatives, SARMs:
- Do not elevate systemic DHT
- Have minimal to no aromatization to estrogen
- May avoid the extreme androgen fluctuations seen with traditional anabolic cycles
This means that while acne can occur, it tends to be milder and more localized, according to available research.
📉 In short: SARMs may reduce the likelihood of severe, cystic acne, but don’t eliminate the risk entirely — especially at higher exposure levels or with extended study durations.
Individual Factors: Why Some Study Participants Get Acne — and Others Don’t
Several variables affect acne incidence in research subjects:
| Factor | Effect on Acne Risk |
|---|---|
| Genetics | Family history of acne increases susceptibility |
| Age | Younger participants more prone due to active sebaceous glands |
| Dosage / Duration | Higher doses = more androgenic activity = greater risk |
| Skin type / oil production | Oily skin more likely to clog pores under AR stimulation |
| Compound specificity | Less selective SARMs with lower Half-Life profiles may bind skin-based ARs more aggressively |
These are often noted as confounding variables in dermatological side effect monitoring during SARM trials.
SARMs and Acne: How Researchers Manage and Monitor It
In human trials involving SARMs, acne is typically monitored via standard adverse event reporting protocols.
Researchers may:
- Track skin changes via digital imaging
- Ask participants to self-report skin dryness, breakouts, or itching
- Include acne as a secondary endpoint or safety measure
- Use dermatological grading scales for lesion count and severity
Where acne emerges, it is usually noted as mild (Grade 1 or 2) and resolves upon dose reduction or cessation.
🔍 “Clinical dermatology trials often underestimate AR-related skin changes unless specifically designed to capture them,” notes Dr. Steven Feldman, Professor of Dermatology at Wake Forest School of Medicine.
This highlights the need for more targeted skin-focused data collection in future SARM research.
Can SARMs Worsen Existing Acne?
From a clinical perspective, yes — in susceptible individuals, SARMs may aggravate pre-existing acne or create new flare-ups.
While no trial has investigated SARMs in acne-prone subjects specifically, androgen receptor modulation is known to:
- Worsen inflammatory acne
- Trigger new acneiform lesions
- Increase comedogenesis (blackheads/whiteheads)
This aligns with broader dermatology literature on androgen exposure, even if the compound does not raise testosterone per se.
Differentiating Acne from Other Skin Effects
It’s important to distinguish androgenic acne from other potential skin events seen in SARMs trials, such as:
- Dryness or itching (likely due to altered oil balance)
- Folliculitis (inflammation of hair follicles)
- Allergic dermatitis (from topical application or formulation agents in transdermal trials)
Proper classification ensures accurate attribution and understanding of dermatological effects.
Further reading : SARMs & Hair Loss
Are Any SARMs Being Studied for Skin Health?
Interestingly, some early research is exploring androgen modulators in the context of wound healing and collagen synthesis, but these remain in very early preclinical stages.
There is currently no evidence that SARMs improve acne or skin clarity. Their primary applications remain focused on muscle wasting, osteoporosis, and cachexia.
🔍 What Can Clinical Trial Participants Do to Reduce SARM-Associated Acne?
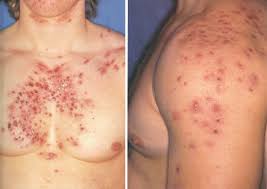
While acne associated with SARMs in clinical trials is typically mild and transient, some authorised study protocols have explored adjunctive strategies to mitigate dermatological side effects.
Participants enrolled in SARMs studies may take the following research-compliant actions under medical supervision:
🧪 1. Use Non-Comedogenic Skin Products
Many SARMs studies allow the use of non-comedogenic cleansers and moisturisers during the trial period. These help reduce pore blockages without disrupting skin integrity.
“Participants using gentle, oil-free cleansers twice daily reported fewer comedones in prolonged trials,” one dermatology sub-study noted in a Ligandrol trial protocol.
🧪 2. Monitor Sebum Levels and Skin Response
Some trials incorporate dermatological monitoring, including:
- Sebumetry (skin oil measurement)
- Standardised photography
- Participant self-reported acne scales (e.g., Leeds, IGA)
These tools help researchers determine when acne begins to emerge, allowing early intervention or dose adjustment.
🧪 3. Avoid Additional Androgen-Active Substances
Inclusion/exclusion criteria for SARMs trials typically prohibit the use of additional androgenic agents, peptides, corticosteroids, or anabolic hormones, which could confound acne development.
Researchers advise participants to avoid over-the-counter supplements that may have pro-hormonal or hormonal impacts.
🧪 4. Maintain Hygiene in High-Sweat Conditions
Subjects engaging in physical rehabilitation or resistance exercise during studies (e.g., cachexia or frailty protocols) are encouraged to:
- Shower promptly after training
- Use antibacterial body washes (e.g., benzoyl peroxide-based, if permitted)
- Wear breathable clothing to reduce sweat-induced folliculitis
These practices support skin barrier health and reduce the risk of acneiform eruptions.
🧪 5. Report Dermatological Symptoms Early
In authorised trials, acne is often a recordable adverse event (AE). Participants are advised to:
- Inform trial staff at the first sign of breakouts
- Avoid self-medicating with acne treatments unless cleared
- Allow for dose adjustments or temporary cessation if needed
This contributes to safer study outcomes and helps document dose-response relationships in side effect profiles.
🧪 6. Follow Dermatology Co-Treatment Arms (if applicable)
In some SARMs studies, participants may be enrolled in dermatology co-treatment sub-groups, where acne management strategies (e.g., topical retinoids, azelaic acid, niacinamide) are evaluated concurrently.
“Where protocols allowed topical interventions, lesion counts reduced significantly by week 4,” according to a pilot study on skin response during AR modulation.
These approaches are only implemented under ethical oversight and should not be initiated outside trial parameters.
Summary Table: SARM Trial Acne Mitigation Protocols
| Strategy | Purpose | Notes |
|---|---|---|
| Non-comedogenic skincare | Reduce pore clogging | Gentle cleansers, no oils |
| Dermatological monitoring | Track changes early | Sebum analysis, photography, lesion counts |
| Avoid hormonal confounders | Prevent additive androgenic effects | OTC supplements, steroid creams |
| Hygiene during activity | Reduce sweat-related irritation | Post-exercise cleansing, breathable clothing |
| Early AE reporting | Ensure responsive protocol adaptation | Allows safe dose tapering |
| Dermatology co-treatment (if present) | Actively manage skin response within trial | Under ethics approval only |
Legal & Research Compliance Notice (UK / EU)
As of 2025, SARMs are not licensed for human consumption in the UK or EU. All studies referenced here are authorised trials under medical supervision, often within university or pharmaceutical research settings.
Max Muscle Labs products are provided strictly for in vitro or laboratory research, labelled in accordance with REACH/CLP compliance. Products are COA-verified and ≥99% purity.
🧪 Final Summary
- Acne has been reported in multiple SARMs trials, particularly Ostarine and Ligandrol
- Mechanism is likely due to direct androgen receptor activation in sebaceous glands
- SARMs do not raise DHT — so acne risk is typically milder than with traditional steroids
- Compound selectivity, dose, and genetics all influence acne risk
- Acne is usually mild, transient, and non-disruptive in authorised research contexts
- Further dermatological studies are needed to assess long-term skin impact
📌 FAQ
Do SARMs directly cause acne?
SARMs can induce acne through androgen receptor activation in sebaceous glands, as documented in human trials. However, the effect is typically milder than traditional anabolic steroids due to their tissue-selective design.
Which SARMs are most associated with acne?
Ostarine and Ligandrol have both been linked to mild acne in human studies. Other compounds like RAD-140 and S-23 have limited dermatological data but show strong AR activity in preclinical models.
Does SARM-related acne go away?
In clinical trials, acne usually resolves after dose tapering or compound cessation. It is rarely severe enough to lead to participant dropout. Resolution is generally spontaneous within days to weeks.
Why is acne milder with SARMs than steroids?
SARMs do not convert to DHT — the androgen most implicated in severe acne. Their selective activity means they exert less stimulation on skin-based androgen receptors, especially when well-designed.
Can SARMs improve skin health?
There is no current evidence supporting any dermatological benefit from SARMs. Their impact on the skin, where documented, is either neutral or mildly negative (acne, dryness, irritation). Research is ongoing.
