What authorised research tells us about testicular atrophy and androgen modulation
TL;DR
Yes, authorised clinical studies and preclinical trials suggest that SARMs can reduce testicular size, primarily through suppression of luteinizing hormone (LH) and testosterone production. This testicular shrinkage is generally temporary and reversible in post-trial recovery periods, but highlights the need for careful hormonal monitoring in clinical settings.
🔑 Key Takeaways
- SARMs can suppress natural testosterone production by interfering with the hypothalamic–pituitary–testicular axis (HPTA), even though they are tissue-selective.
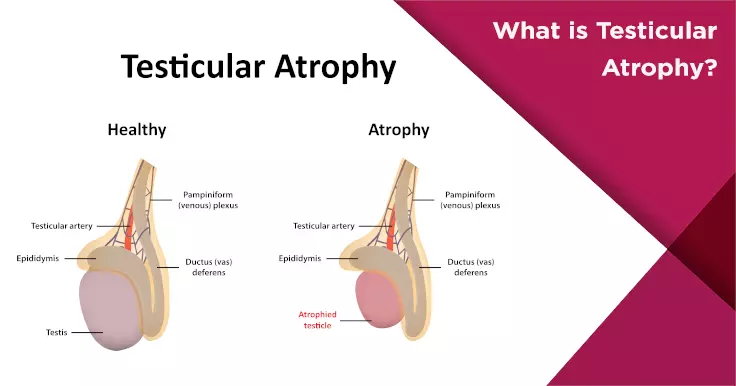
- Testicular atrophy (shrinkage) observed in research settings is not due to direct damage, but rather reduced LH and FSH levels, which diminish testicular stimulation.
- Human trials, such as those involving LGD-4033, have shown significant hormonal suppression even at low doses (e.g., 1 mg/day for 21 days), supporting the potential for testicular impact.
- Animal studies (e.g., with S-23) confirm dose-dependent reductions in testicular size and sperm production, making some SARMs candidates for male contraceptive research.
- The effect is generally reversible, with endocrine function and testicular volume often returning to baseline post-administration during the recovery phase of authorised studies.
- Post-trial recovery protocols (clinical equivalent to “PCT”) may include Clomiphene or Enclomiphene, used under strict medical supervision to restore hormonal balance.
- These findings underline the importance of careful hormonal monitoring, ethical oversight, and follow-up in any authorised research involving SARMs or other androgen modulator
SARMs: Targeted Action, Systemic Impact
Selective Androgen Receptor Modulators (SARMs) are being investigated for their tissue-selective anabolic properties — aiming to promote muscle growth, bone density, and fat loss while avoiding the systemic side effects of traditional anabolic steroids.
But while SARMs avoid conversion to estrogen or dihydrotestosterone (DHT), they can still interfere with the body’s endocrine feedback loop — particularly the hypothalamic–pituitary–testicular axis (HPTA).
In clinical terms, this disruption may reduce testicular stimulation, leading to a measurable decrease in testicle volume in some participants.
How Testicular Atrophy Occurs in Research Models
The biological mechanism behind testicular size reduction is consistent with other forms of exogenous androgen modulation.
“Exogenous activation of androgen receptors in skeletal muscle reduces the need for endogenous testosterone production, triggering negative feedback at the level of the hypothalamus and pituitary.”
— NCBI
This leads to:
- Suppressed gonadotropin-releasing hormone (GnRH) from the hypothalamus
- Reduced luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary
- Decreased testosterone and sperm production within the testes
- Observable testicular atrophy over time
This mechanism has been consistently demonstrated in both animal models and early-phase human trials.
Evidence from Authorised Trials
📌 LGD-4033 (Ligandrol) — Human Study
A double-blind, placebo-controlled Phase I trial published in Journals of Gerontology (Basaria et al., 2013) evaluated the effects of LGD-4033 on healthy men over 21 days.
- Suppression of total testosterone: up to 55%
- Reduction in LH and FSH: dose-dependent
- Testicular volume: not directly measured, but hormonal profile indicates expected atrophic response
📌 S-23 — Preclinical Model
In rodent models, S-23 produced nearly complete suppression of spermatogenesis and significant reduction in testicular weight. These findings have made S-23 a candidate for male contraceptive research.
- Study: Jones et al., 2010, Biology of Reproduction
- Reported dose-dependent testicular atrophy
- Recovery observed post-administration in controlled phases
Which SARMs Show Highest Atrophic Potential?
Different SARMs vary significantly in their suppressive potential. Based on published data and animal trials, the following hierarchy can be outlined:
| SARM Compound | Androgenic Suppression Potential | Evidence of Atrophy |
|---|---|---|
| S-23 | Very High | Confirmed in rodent trials |
| RAD-140 | High | Hormonal suppression observed (preclinical) |
| LGD-4033 | High | Human suppression trial data |
| YK-11 | High (also affects DHT pathways) | Not fully characterised |
| MK-2866 (Ostarine) | Moderate | Minimal atrophy in shorter trials |
| S4 (Andarine) | Moderate | Mild suppression effects |
Note: Direct measurements of testicular size are limited in human trials; most conclusions are extrapolated from hormone profiles and animal histology.
Is the Effect Reversible?
Yes, in most research settings, testicular shrinkage has been shown to reverse after the discontinuation of the SARM under investigation.
For instance, in post-trial recovery periods:
- LH and FSH levels rebounded
- Endogenous testosterone production resumed
- Testicular volume returned to baseline in preclinical observations
“Recovery of endogenous hormone levels occurred within weeks to months after cessation of treatment, depending on dose and duration.”
— Pharmacology & Therapeutics, 2021
However, longer or higher-dose exposures may prolong recovery — which is why researchers often recommend structured post-trial monitoring and endocrine function assessment.
Post-Trial Hormonal Recovery: The Clinical Equivalent of PCT
In fitness communities, the concept of Post Cycle Therapy (PCT) is often discussed as a means to restore natural hormone levels. In clinical research, however, the equivalent process involves:
- Endocrine follow-up protocols
- Use of Selective Estrogen Receptor Modulators (SERMs) like Clomiphene or Enclomiphene under physician guidance
- Monitoring of testosterone, LH, FSH, and sperm parameters
These interventions are only used under authorised trial protocols, and are not recommended outside regulated environments.
Risk Management in Research Settings
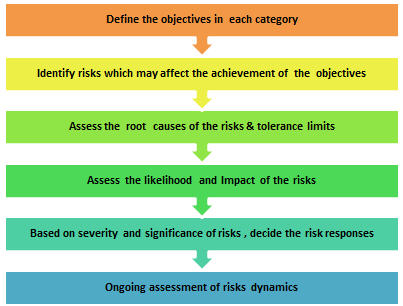
To mitigate hormonal side effects like testicular atrophy, authorised trials often implement the following protocols:
✅ Short trial durations (e.g. 14–28 days)
✅ Dose titration based on preclinical toxicity data
✅ Baseline and follow-up hormone testing
✅ Exclusion of participants with endocrine disorders
✅ Post-trial recovery observation periods
These controls are essential to protect participant health and ensure valid interpretation of endocrine biomarkers.
FAQ: Research-Oriented Clarifications
Q: Can SARMs cause testicular shrinkage in human trials?
Yes. Studies such as those involving LGD-4033 have shown suppression of testosterone and LH, which are associated with testicular atrophy.
Q: Is the effect permanent?
Current data suggest the effect is temporary with recovery observed post-administration in both animals and humans.
Q: Why aren’t testicles always directly measured in human studies?
Due to ethical and logistical reasons, most human SARM trials rely on biochemical markers (e.g., LH, testosterone) rather than physical testicular measurements.
Q: Are post-trial recovery protocols the same as PCT in bodybuilding circles?
Not exactly. While they may use similar compounds (e.g., SERMs), post-trial interventions are medically supervised and based on standardised clinical protocols.
Final Thoughts: What This Means for Future Research
Testicular atrophy in SARM research isn’t unexpected — it’s a well-understood outcome of androgen modulation. However, under controlled clinical conditions, it is:
- Predictable
- Measurable
- Reversible
For researchers, this underscores the importance of baseline hormone profiling, follow-up testing, and clear ethical frameworks when studying any compound with androgenic activity.
The ultimate goal of SARMs development is not just effectiveness — but safety. Understanding and managing hormonal suppression is a core part of that mission.
