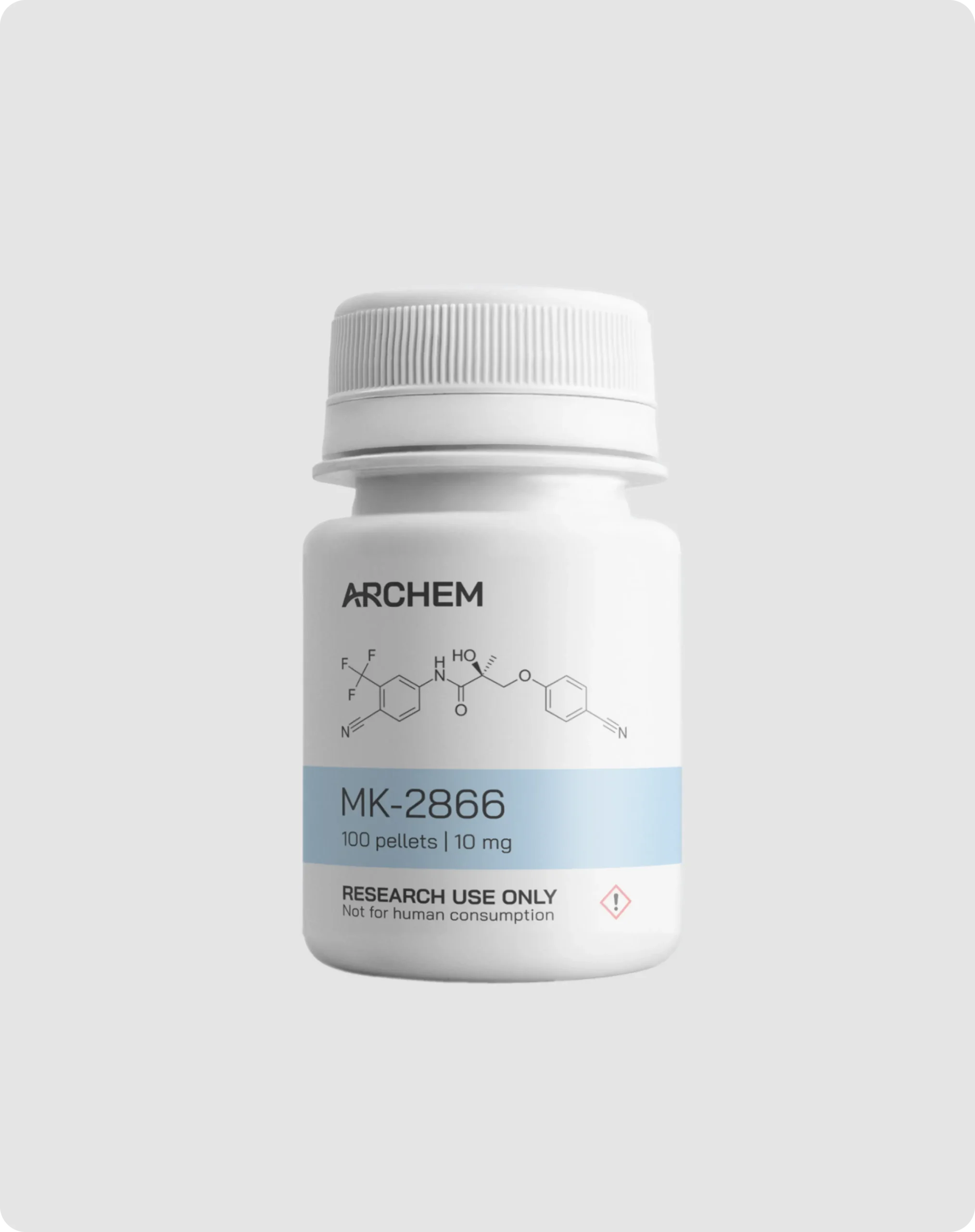
Description
SAFETY DATA SHEET
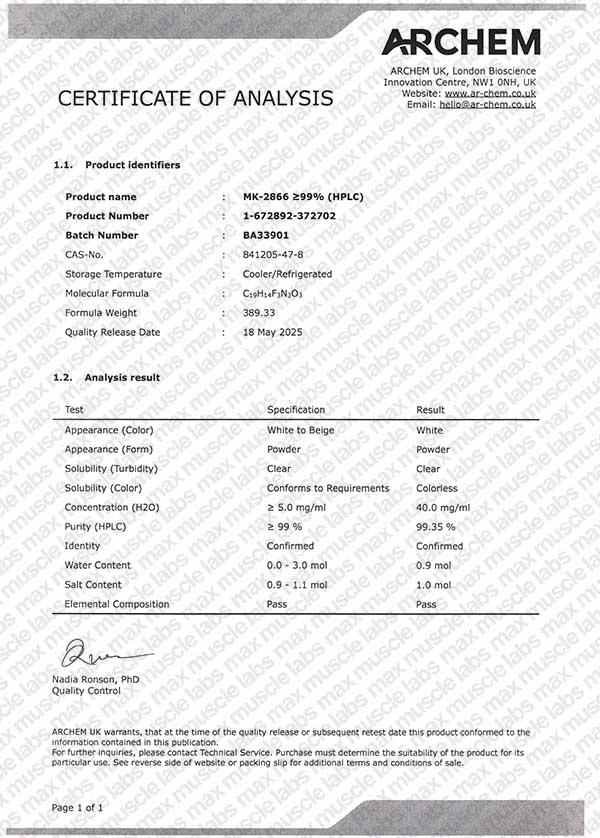
MK-2866 HPLC BA33901
MK-2866 COA BA33901
Ostarine, chemically designated as MK-2866, is a non-steroidal Selective Androgen Receptor Modulator (SARM) developed to prevent and treat muscle wasting and osteoporosis. Its mechanism of action involves high-affinity binding to androgen receptors in skeletal muscle and bone tissues, selectively stimulating anabolic activity without the androgenic side effects commonly associated with traditional anabolic steroids.
- Demonstrates anabolic activity in skeletal muscle tissue models, including under conditions lacking mechanical load stimulation
- Modulates bone remodeling pathways, indicating potential utility in preclinical models of reduced bone mineral density (e.g., osteopenia, osteoporosis)
- Exhibits muscle-preserving effects under simulated states of caloric deficit or musculoskeletal disuse, relevant to models of cachexia and immobilization
Its tissue-selective profile minimizes undesirable effects on the prostate or other non-target organs. Ostarine does not undergo aromatization to estrogen and exhibits minimal hepatotoxicity, which contributes to its favorable safety profile in short-term research applications.
CAS Number: 841205-47-8
Molecular formula: C19H14F3N3O3
Molar mass: 389.33 g/mol
Purity: ≥99% (see HPLC and COA documentation)
Concentration: 10 mg
Half-life: ~24 hours
Storage: 2 years at 20°C
⚠ Important Note: This product is supplied strictly for laboratory research use only. It is not approved for human consumption or therapeutic use. Not intended to diagnose, treat, cure, or prevent any disease.
Frequently Asked Questions about Ostarine
Ostarine (MK-2866) — Frequently Asked Questions
1. What is Ostarine (MK-2866)?
Ostarine, also known as MK-2866 or Enobosarm, is a Selective Androgen Receptor Modulator (SARM) developed to mimic some effects of testosterone in muscle and bone tissue.
In research settings, it’s studied for its potential to support muscle retention, lean mass, and bone health without the androgenic effects of traditional steroids.
2. Is Ostarine legal to buy in the UK?
Ostarine is not licensed for human use in the UK or EU.
However, it is legal to purchase and possess for laboratory research when supplied by registered research-chemical vendors.
Max Muscle Labs supplies Ostarine strictly for research purposes only, in accordance with UK and EU compliance standards.
3. What purity level does your Ostarine have?
Each batch of MK-2866 is independently tested using HPLC (High-Performance Liquid Chromatography) to confirm purity, typically above 99%.
You can verify your specific bottle by checking the batch number printed on the bottom and viewing its COA (Certificate of Analysis) on our COA page.
4. How do researchers store Ostarine correctly?
For research stability, Ostarine should be stored:
— In a cool, dry environment (ideally 2–8 °C)
— Away from direct sunlight or moisture
— With the lid tightly sealed to prevent oxidation
Following these conditions preserves compound integrity over time.
Further reading : HSE chemical storage guidance
5. How does Ostarine differ from RAD-140 or LGD-4033?
While all three are SARMs, Ostarine (MK-2866) is generally considered milder and better suited for research into muscle preservation, while RAD-140 and LGD-4033 are often studied for anabolic potency and strength adaptation.
Each compound interacts with the androgen receptor differently — meaning comparative results depend on study design and tissue selectivity.
Further information: Ostarine and it’s related research
6. Can Ostarine be used for human consumption?
No.
Ostarine (MK-2866) is not approved for human use by the MHRA, FDA, or EMA.
All products sold by Max Muscle Labs are for laboratory research only and are not intended for dietary or medical use.
Read our guidance here
7. Where can I verify testing data?
Visit our COA & HPLC Testing page.
Enter the batch number printed on your bottle to view the full laboratory report, including HPLC chromatogram and purity results.
8. How often are COAs updated?
COAs and HPLC reports are updated for every new production batch.
Older results remain archived for transparency, so researchers can compare data across different lots for consistency.
9. What does the COA show?
A COA (Certificate of Analysis) includes:
— Compound identity (e.g., Ostarine MK-2866)
— Batch number
— Purity percentage
— Testing method (HPLC / NMR / LC-MS)
— Independent lab signature and date
It’s the definitive verification that your sample matches its specification.






Mo (verified purchase) –
The delivery was okay, but it took a while. The product is good.
MML Support (store manager) –
Hi Mo,
Glad to hear you’re happy with the product. Sorry again for the delay – we’ve had a higher-than-usual volume of orders recently, and a few shipments took longer than expected.
That said, we’re actively scaling our logistics to keep up and ensure faster turnarounds moving forward.
Thanks again for your patience and support.
Nigel (verified purchase) –
As described.
Harry (verified purchase) –
My experience was super good with support team
Max (verified purchase) –
Works well ! Fast delivery to Europe
Justin (verified purchase) –
Easy payment. Quick shipping. Seems to be a quality product. Thank you
Peter (verified purchase) –
Great compound
Mark (verified purchase) –
Cheaper than other sites and good quality product.
George (verified purchase) –
Solid product
Brian (verified purchase) –
Dosent disappoint great quality
richard (verified purchase) –
good
MML Support (store manager) –
Hi Richard,
Thanks for your feedback — we appreciate you taking the time to leave a review.
If there’s anything we can do to make your experience a 5-star one next time, feel free to let us know!
Zach (verified purchase) –
The lab experiment is going great
Paul (verified purchase) –
Haven’t used yet but arrived just fine
Michael (verified purchase) –
Zero complaints. Discreet, and fast shipping, great customer service as well.
Ethan (verified purchase) –
Really working well
Rob (verified purchase) –
top service and quality. had an issue with my order but they sorted it quickly
Eric (verified purchase) –
Nice quality
jason (verified purchase) –
Legit
Justin (verified purchase) –
product purity was superb
Ben (verified purchase) –
very effective. prompt shipping
Chris (verified purchase) –
Top notch
David (verified purchase) –
Great